Organised and funded by GSK.
For UK healthcare professionals.
This site contains promotional material.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or UKSafety@gsk.com
Be part of the illuminate community today, register below.
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
To stay up to date with the latest peer-led, practical resources designed to support your professional development and the implementation of immunisation programmes, please register.
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminateYou seem to be interested in what illuminate has to offer... why not register to stay updated
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
To stay up to date with the latest peer-led, practical resources designed to support your professional development and the implementation of immunisation programmes, please register.
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminate
This website is organised and funded by GSK. Adverse event reporting and prescribing information can be found below the registration form.
No eligible patient should miss out on the opportunity to be protected from shingles.
3 actions you can take to maximise Shingles Awareness Week 2024 (26th Feb – 3rd March)
Dedicated Clinics
Plan dedicated shingles clinics during Shingles Awareness Week
Proactive Call & Recall
Send additional invites or reminders during Shingles Awareness Week
Engage with our supportive content!
Practical resources & webinars designed to support your programme implementation
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
Register to stay updated with the latest peer-led, practical resources designed to support programme implementation!
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminateAdverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441
For the GB and NI SHINGRIX® (herpes zoster vaccine, recombinant, adjuvanted) prescribing information, click here.
Welcome to Peers in Practice
For UK healthcare professionals — information and support on healthcare challenges and primary care issues
Important notice: This content is intended for UK healthcare professionals.
By entering this site, you are confirming you are a UK healthcare professional. The HCP Clinical Hub section of this site contains promotional information.
If you are not a healthcare professional, please select ‘I’m a member of the UK public’.
You are now leaving this GSK website to visit a third-party website that is not owned or controlled by GSK. GSK does not recommend, endorse or accept liability for websites controlled by third-parties.
Log in to access this content
This content is for UK healthcare professionals only. If you have already registered, enter your email address below to log in.
Not registered? Register now to access this content.

Organised and funded by GSK. For UK healthcare professionals only. This site contains promotional material.
Welcome to illuminate
For UK healthcare professionals – this site contains promotional information.
Important notice: This site is intended for UK healthcare professionals. By entering this site, you are confirming that you are a UK healthcare professional.
This site is intended for members of the UK public, patients and their carers.

Organised and funded by GSK. For UK healthcare professionals only. This site contains promotional material.
Welcome to illuminate
For UK healthcare professionals – This section of the site contains promotional information. Please confirm you understand this by clicking the button below.
Important notice: This site is intended for UK healthcare professionals. By entering this site, you are confirming that you are a UK healthcare professional.
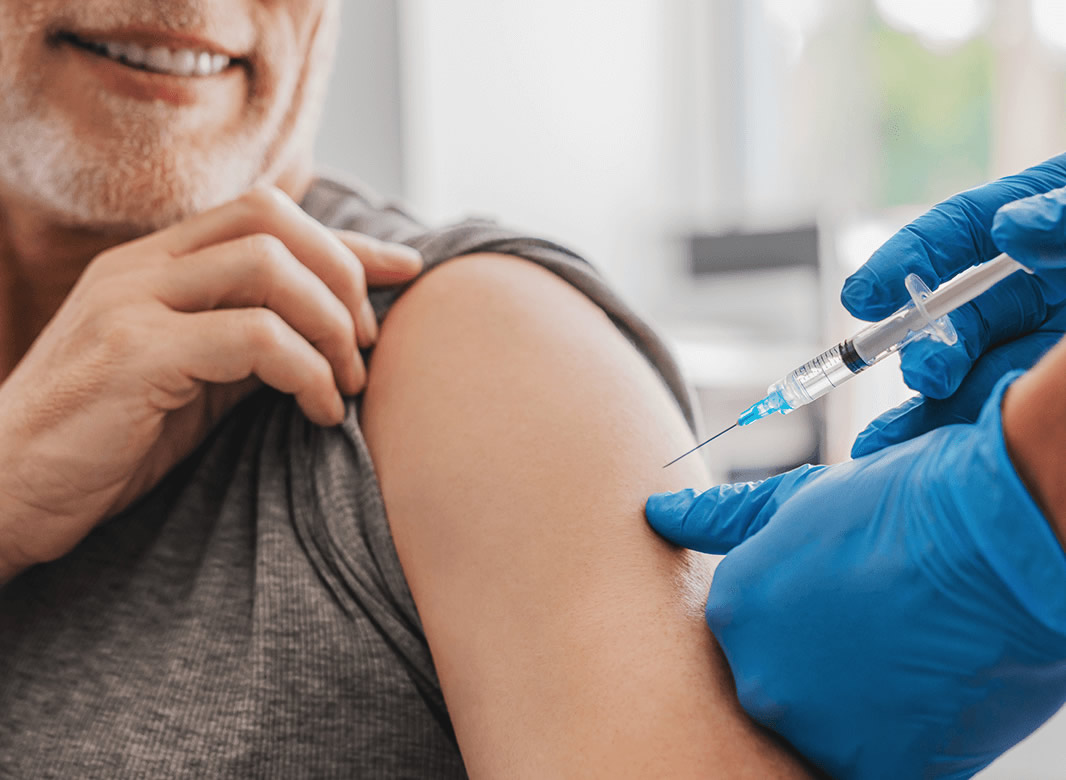
This website is organised and funded by GSK. Adverse event reporting and prescribing information can be found below the registration form.
Your patients attending winter vaccination clinics may also be eligible for non-seasonal shingles vaccination
How can you proactively plan for non-seasonal shingles vaccination, during the winter period?
Organise & implement dedicated clinics
Highlight the schedule consists of two-doses
Engage with illuminate’s practical resources
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
Register to stay updated with the latest peer-led, practical resources designed to support programme implementation!
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminateAdverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or UKSafety@gsk.com.
For the GB and NI SHINGRIX® (herpes zoster vaccine, recombinant, adjuvanted) prescribing information, click here.
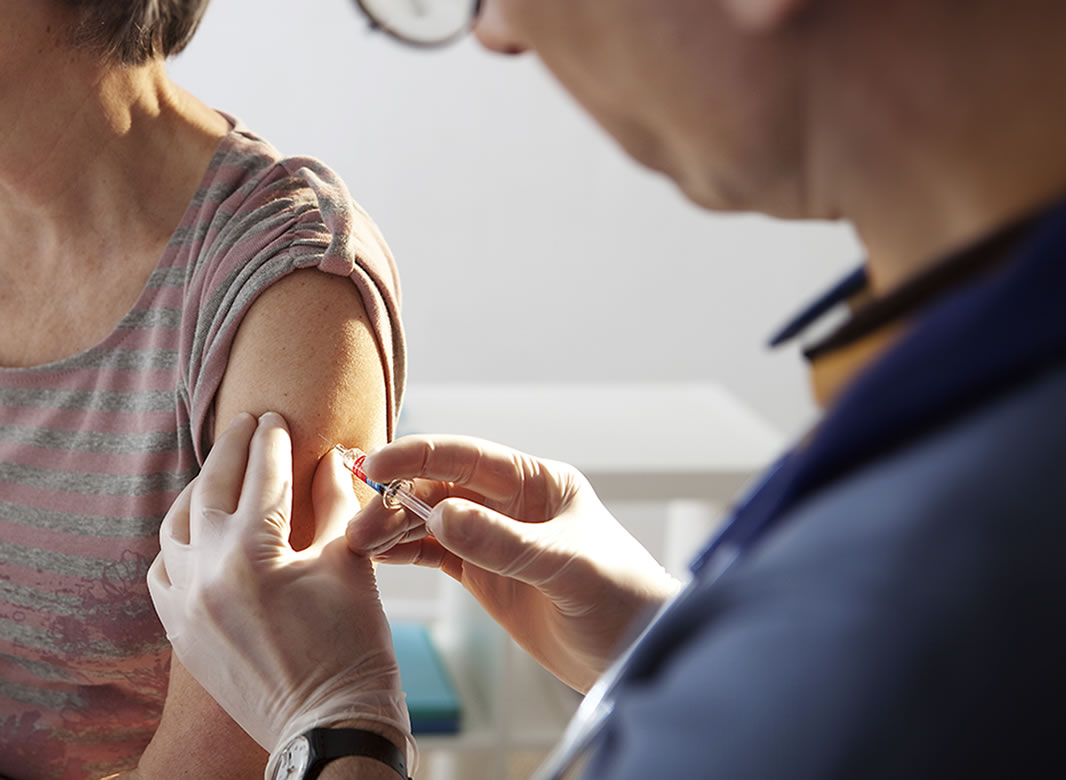
This website is organised and funded by GSK. Adverse event reporting and prescribing information can be found below the registration form.
Your patients attending winter vaccination clinics may also be eligible for non-seasonal shingles vaccination
How can you proactively plan for non-seasonal shingles vaccination, during the winter period?
Organise & implement dedicated clinics
Highlight the schedule consists of two-doses
Engage with illuminate’s practical resources
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
Register to stay updated with the latest peer-led, practical resources designed to support programme implementation!
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminateAdverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or UKSafety@gsk.com.
For the GB and NI SHINGRIX® (herpes zoster vaccine, recombinant, adjuvanted) prescribing information, click here.

This website is organised and funded by GSK. Adverse event reporting and prescribing information can be found below the registration form.
One year on… are you continuing to seize the opportunity to help protect your patients from shingles?
Access peer-led, solution focussed resources
Support your understanding of the Shingles National Immunisation Programme criteria
Simplify the planning and implementation of vaccination clinics
Learn from your peers and drive immunisation best practice
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
Register to stay updated with the latest peer-led, practical resources designed to support programme implementation!
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminateAdverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or UKSafety@gsk.com.
For the GB and NI SHINGRIX® (herpes zoster vaccine, recombinant, adjuvanted) prescribing information, click here.
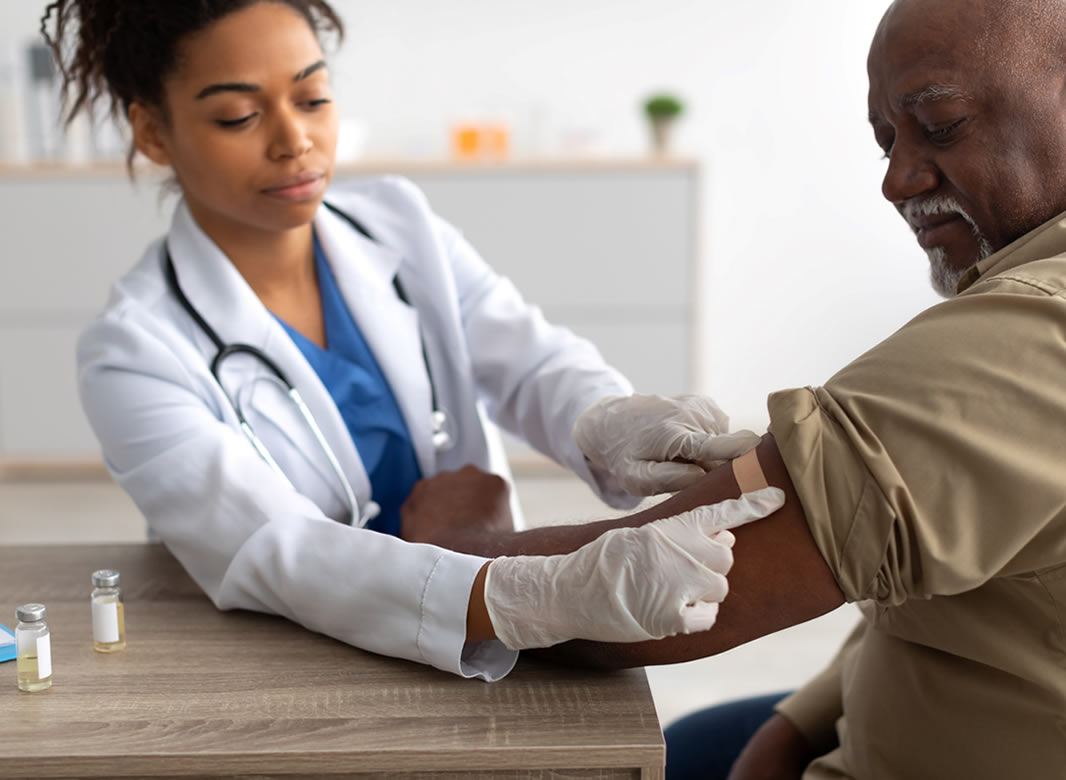
This website is organised and funded by GSK. Adverse event reporting and prescribing information can be found below the registration form.
Are you on track with shingles vaccination in your practice?
Maximise shingles immunity by ensuring completion of the full Two-Dose Vaccination Schedule

Plan and Implement dedicated Shingles clinics

Identify and proactively recall first and second dose patients for the shingles vaccination

Engage with practical resources, like Patient Support Materials, on Illuminate
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
Register to stay updated with the latest peer-led, practical resources designed to support programme implementation!
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminateAdverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or UKSafety@gsk.com.
For the GB and NI SHINGRIX® (herpes zoster vaccine, recombinant, adjuvanted) prescribing information, click here.

Organised and funded by GSK. For UK healthcare professionals only. This site contains promotional material.
Welcome to inhale
For UK healthcare professionals — This section of the site contains promotional information
Important notice: This site is intended for UK healthcare professionals. By entering this site, you are confirming that you are a UK healthcare professional.
This site is intended for members of the UK public, patients and their carers.
November 2024 | NP-GB-FVU-WCNT-240001

This website is organised and funded by GSK. Adverse event reporting and prescribing information can be found below the registration form.
No eligible patient should miss out on the opportunity to be protected from shingles.
3 actions you can take to maximise Shingles Awareness Week 2025 (24th February – 2nd March)
Dedicated Clinics
Plan dedicated shingles clinics during Shingles Awareness Week
Proactive Call & Recall
Send additional invites or reminders during Shingles Awareness Week
Engage with our supportive content!
Practical resources & webinars designed to support your programme implementation
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
Register to stay updated with the latest peer-led, practical resources designed to support programme implementation!
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminateAdverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or UKSafety@gsk.com.
For the SHINGRIX® (herpes zoster vaccine, recombinant, adjuvanted) prescribing information, click here.
Be part of the inhale professional hub today. Register below.
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
To stay up to date with upcoming COPD resources to support your professional development and the implementation of behaviour change principles in your practice, please register.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK on 0800 221 441 or UKSafety@gsk.com.
For the GB and NI Trelegy Ellipta® (fluticasone furoate/umeclidinium/vilanterol) prescribing information Click here.
Thank you for registering
We will be in touch when more resources for you are added to inhale.
Go back to exploring inhaleNovember 2024 | PM-GB-CPU-WCNT-240019
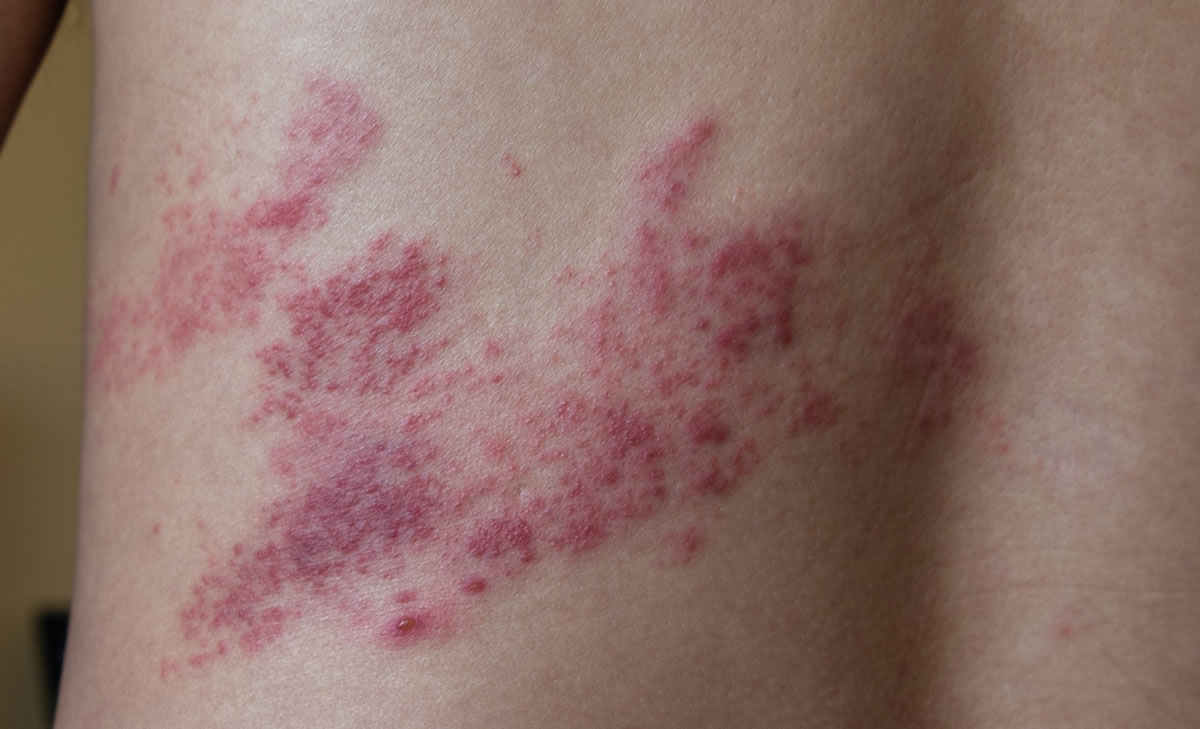
This website is organised and funded by GSK. Adverse event reporting and prescribing information can be found below the registration form.
Maximise the summer period to help protect your eligible patients against shingles
Vaccine uptake data for the Shingles National Immunisation Programme in England1,2
Immunocompetent
65-year-olds
32.6%
Of the estimated 158,166 adults who turned 65 between 1st Sept 2023 and 30th Nov 2023 had received their first dose by 23rd July 2024
Immunocompetent
70-year-olds
43.0%
Of the estimated 128,220 adults who turned 70 between 1st Sept 2023 and 30th Nov 2023 had received their first dose by 23rd July 2024
- UK Health Security Agency. Shingrix vaccine uptake report (adults eligible from September 2023 to May 2024 and vaccinated to the end of July 2024): England. Updated April 2025 Available at: https://www.gov.uk/government/publications/shingles-immunisation-programme-shingrix-evaluation-reports/596b592d-b05d-4558-b5f4-7e2c2cb51e3c (Last Accessed: June 2025)
- UK Health Security Agency. Shingles Immunisation Programme [SHINGRIX]: Evaluation Reports. Shingrix vaccine coverage by ICB and LA (England), September 2023 to February 2024 (vaccinated to end-April 2024). Updated April 2025. Available at: https://www.gov.uk/government/publications/shingles-immunisation-programme-shingrix-evaluation-reports (Last Accessed: June 2025)
Organised and funded by GSK. For UK healthcare professionals. This site contains promotional material.
Register to stay updated with the latest peer-led, practical resources designed to support programme implementation!
Thank you for registering
We will be in touch when more resources for you are added to illuminate!
Go back to exploring illuminateAdverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or UKSafety@gsk.com.
For the SHINGRIX® (herpes zoster vaccine, recombinant, adjuvanted) prescribing information, click here.