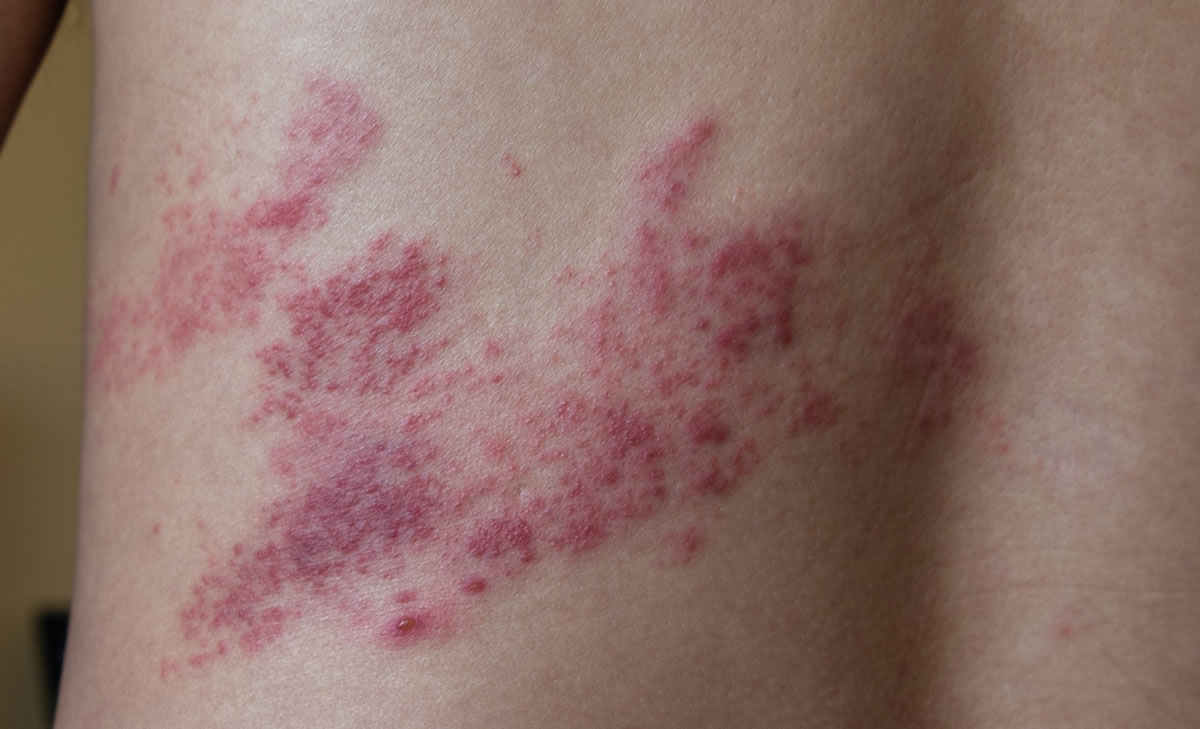
Eligibility Checker – Shingles Vaccination
Use our Shingles Vaccination Eligibility Checker to support you in determining whether your patients are eligible for their shingles vaccination, under the National Immunisation Programme.
Adverse events reporting and prescribing information can be found at the bottom of the page.
Healthcare professionals have been consulted by GSK and received honorarium.
Shingles Vaccination Eligibility Checker
Enter your patient's date of birth, and requested information below to find out if they are currently eligible for shingles vaccination on the National Immunisation Programme. Both questions are to be answered to generate a result providing guidance on eligibility.
Please note, results are based on the guidance for NHS England (UKHSA Green Book: Shingles (herpes zoster): the green book chapter). Guidance varies in Northern Ireland, Scotland and Wales.
This eligibility checker is intended to support healthcare professionals (HCPs) in identifying patients that may be eligible for shingles vaccination. The checker does not make clinical decisions and is not intended to replace individual clinical judgement of the HCP utilising the checker. The National Guidelines (UKHSA Green Book: Shingles (herpes zoster): the green book chapter) and Summary of Product Characteristics should be consulted when making treatment decisions for individual patient management.
This checker is designed to assess the eligibility of patients who have not yet received shingles vaccination.
There may be a small number of your patients that may have previously received Zostavax (herpes zoster vaccine (live)), and will be eligible for revaccination under the National Immunisation programme - please refer to the National Guidance.
1. What is your patient's date of birth?
2. Have they already received shingles vaccination (either a single dose of Zostavax (zoster vaccine live) or two doses of SHINGRIX)?
2. Does your patient meet any of the below criteria? 1:
- Acute and chronic leukaemia or clinically aggressive lymphoma (including Hodgkin’s lymphomaa), and is less than 12 months since achieving cure
- Under follow up for chronic lymphoproliferative disorders including haematological malignancies such as indolent lymphoma, chronic lymphoid leukaemia, myeloma, Waldenstrom’s macroglobulinemia and other plasma cell dyscrasias (N.B: this list not exhaustive)
- Immunosuppression due to HIV/AIDS with a current CD4 count of below 200 cells/μl
- Primary or acquired cellular and combined immune deficiencies – those with lymphopaenia (<1,000 lymphocytes/μl) or with functional lymphocyte disorder
- Received an allogeneic (cells from a donor) or an autologous (using their own cells) stem cell transplant in the previous 24 months
- Received a stem cell transplant more than 24 months ago but have ongoing immunosuppression or graft versus host disease (GVHD)
- Receiving or have received in the past 6 months immunosuppressive chemotherapy or radiotherapy for any indication
- Receiving or have received in the previous 3 months targeted therapy for autoimmune disease, such as JAK inhibitors or biologic immune modulators including:
- B-cell targeted therapies (including rituximab but for which a 6-month period should be considered immunosuppressive), monoclonal tumor necrosis factor inhibitors (TNFi), T-cell co-stimulation modulators, soluble TNF receptors, interleukin (IL)-6 receptor inhibitors
- IL-17 inhibitors, IL 12/23 inhibitors, IL 23 inhibitors (N.B: this list is not exhaustive)
- Receiving or have received in the previous 6 months immunosuppressive therapy for a solid organ transplant
- Moderate to high dose corticosteroids (equivalent ≥20mg prednisolone per day) for more than 10 days in the previous month
- Long term moderate dose corticosteroids (equivalent to ≥10mg prednisolone per day for more than 4 weeks) in the previous 3 months
- Any non-biological oral immune modulating drugs e.g. methotrexate >20mg per week (oral and subcutaneous), azathioprine >3.0mg/kg/day; 6-mercaptopurine >1.5mg/kg/day, mycophenolate >1g/day) in the previous 3 months
- Certain combination therapies at individual doses lower than stated above, including those on ≥7.5mg prednisolone per day in combination with other immunosuppressants (other than hydroxychloroquine or sulfasalazine) and those receiving methotrexate (any dose) with leflunomide in the previous 3 months
Results
The result will be displayed here, indicating whether your patient is eligible for shingles vaccination under the National Immunisation Programme.

An Overview on Eligibility for the Shingles National Immunisation Programme
From 1st September 2025, the eligibility for severely immunocompromised individuals expanded to aged 18 years and above (with no upper age limit).
Proactive implementation of the programme increases the opportunity to help protect more people and drive the national uptake of the shingles vaccination – are you seizing this opportunity?
Severely Immunosuppressed Individuals2
- Severely immunosuppressed individuals aged 18 years and over who have not received the shingles vaccine before, are eligible for the SHINGRIX (herpes zoster vaccine, recombinant, adjuvanted) vaccine.
- There is no upper age limit, but individuals should be offered the vaccine as soon as they become eligible to provide protection as early as possible.
- Severely immunosuppressed individuals represent the highest priority for vaccination, given their risk of severe disease1.
Older Adults1
- Adults turning 65 years of age (on or after 1st September 2023) are eligible for shingles vaccination.
- Those aged 70-79 years of age who have not yet received shingles vaccination, are already eligible for the shingles vaccine and remain eligible up until their 80th birthday.

Frequently Asked Questions
The patient would not be eligible under the National Immunisation Programme, but will become eligible when they turn 70 years of age.
Please note, this is based on the guidance for NHS England (as per UKHSA Green Book: Shingles (herpes zoster): the green book chapter). Guidance varies in Northern Ireland, Scotland and Wales.
The eligibility of those aged between 66 and 69 depends on when they turned 65.
If an adult is between 65-69 years BEFORE 1st September 2023, they will not be eligible for vaccination under the National Immunisation Programme until they turn 70 years of age.
If they turned 65 years ON OR AFTER 1st September 2023, they remain eligible for vaccination until their 80th birthday.
Please note, this is based on the guidance for NHS England (as per UKHSA Green Book: Shingles (herpes zoster): the green book chapter). Guidance varies in Northern Ireland, Scotland and Wales.

Delivering the Shingles National Immunisation Programme in your practice
For support with programme implementation, access our interactive tools designed to support your practice to take an effective and manageable approach to delivering shingles vaccination.
Implementation ToolsReferences
- UKHSA Green Book: Shingles (herpes zoster): the green book chapter.
- UK Health Security Agency. Shingles vaccination programme: expansion of Shingrix vaccine eligibility to all those who are severely immunosuppressed and aged 18 years and over. Published July 2025.
August 2025 | PM-GB-AVU-WCNT-230009 (V4.0)
For the SHINGRIX® (herpes zoster vaccine, recombinant, adjuvanted) prescribing information, click here.